Background
iPSCs are considered a revolutionary discovery in regenerative medicine for various reasons. One of the most significant advantages of iPSCs is their ability to be generated from a patient's cells (Figure 1), which eliminates the risk of immune-rejection and allows for the creation of patient-specific cells that can be used for personalized medicine in regenerating injured or degenerated tissues. One major challenge in translating the iPSC-based therapy to clinical treatment is the lack of a scalable, reproducible cell manufacturing platform for expanding iPSCs and then differentiating them into targeted cell types for therapeutic treatment. The traditional two-dimensional (2D) culture system has limited scalability; thus, it is challenging to generate the number of cells required for use in clinical therapy. The suspension culture of iPSCs using stirred tank bioreactors is scalable, but it has complex technical procedures. Moreover, it is not suitable for the subsequent differentiation of the expanded iPSCs to target cells. To overcome the manufacturing challenges in iPSC-based therapies, SwRI is developing a novel, scalable, and reproducible iPSC expansion and differentiation system based on the SwRI bioreactor technology developed in a previous IR&D program.
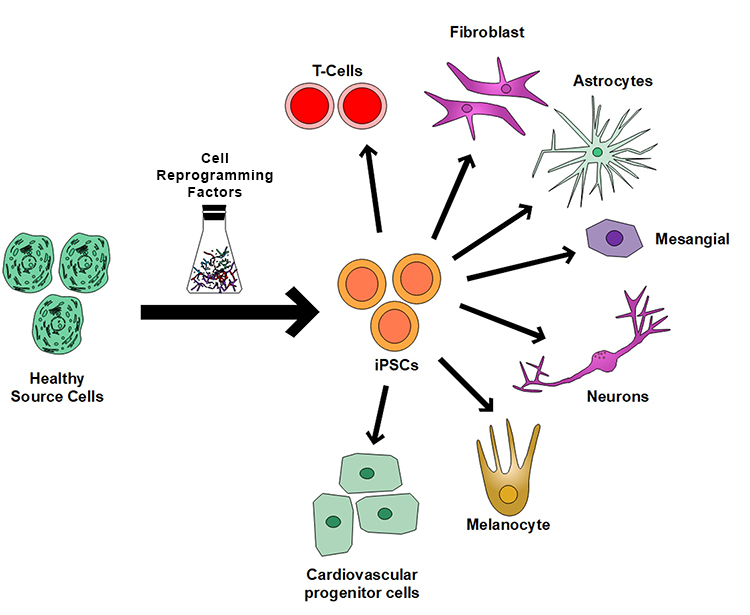
Figure 1: Healthy cells in our body can be programmed back into pluripotent iPSCs, which can then be converted into any types of cells in our body for therapeutic applications.
Approach
SwRI has developed an iPSC expansion process in the novel SwRI bioreactor (Figure 2) system and is working to demonstrate the scalability of iPSC expansion. The project team is developing two methods to differentiate iPSCs into NPCs using the current SwRI bioreactor structure and a new, modified one. Once finished, the team will integrate the iPSCs expansion and differentiation processes to produce NPCs using the SwRI bioreactor system. The team expects the system to produce a clinically relevant population of over 100 million NPCs within 4 weeks.

Figure 2: (a) SwRI’s proprietary 3D cell culture bioreactor is composed of tightly packed interconnected spherical voids in 3D space. (b) The bioreactor is fabricated using 3D printing. (c) The bioreactor facilitates pump-driven perfusion-based cell culture. (d) Human iPSCs reseeded after culturing in SwRI Bioreactor.
Accomplishments
Expansion within the SwRI bioreactor has been successfully demonstrated on a B-250 platform (250 cm2 surface area). This expansion process was directly compared to cells grown in traditional 2D culture conditions (Controls). The results of the expansion process within the bioreactor showed a 6.6-fold increase in cell population within 3 days of proliferation. Furthermore, cells harvested from the bioreactor expansion process underwent analysis using flow cytometry.
This analysis aimed to assess stemness markers and tri-lineage differentiation capabilities and compare them directly to control cells. The initial findings show cells harvested from the bioreactor exhibited characteristics that are directly comparable to those of the 2D control cells in terms of stemness markers and tri-lineage differentiation capabilities.

Figure 3: Flow cytometry results indicating the cells harvested from the bioreactor and the cells harvested from a control flask perform similarly when stained with their respective antibodies. Top row: Mesoderm stained with anti-Brachyruy and anti-NCAM/CD56 to indicate the correct cell type. Endoderm stained with anti-SOX17 and anti-CD184 to indicate the correct cell type. Middle row: Ectoderm stained with anti-Pax-6 and anti-Nestin to indicate the correct cell type. Bottom row: Pre-differentiated cells stained with anti-SOX2, anti-Oct-3/4, anti-SSEA-1, anti-SSEA-4 and anti-TRA-1-60 to indicate pre-differentiation.
