Background
Our group has been developing micro-electro-mechanical system (MEMS) gas chromatographs (GCs) for field portability, with the ultimate field portability being a future spaceflight landed mission. We have demonstrated the utility of the MEMS GC devices in a portable, stand-alone format, developed by the University of Michigan (UM), for the analysis of volatile organic compounds (VOCs) – specifically alkanes from pentane to decane (C5 – C10) and 3-methylpentane and cyclohexane. We also connected this field portable MEMS GC to the MAss Spectrometer for Planetary EXploration (MASPEX) for mass spectrometric detection of the above analytes separated on the MEMS GC. We then used a MEMS GC separation column to demonstrate its capability for the separation of large organics including alkanes, chemically derivatized amino and fatty acids, and polycyclic aromatic hydrocarbons (PAHs).
The MEMS GC separation column was placed in a commercial GC to utilize the injector for vaporization of the liquid sample and the oven for heating of the MEMS GC column. The exit of the MEMS GC column was coupled to MASPEX for mass spectrometric detection of the eluting analytes. The chromatogram produced from this separation is displayed in Figure 1.
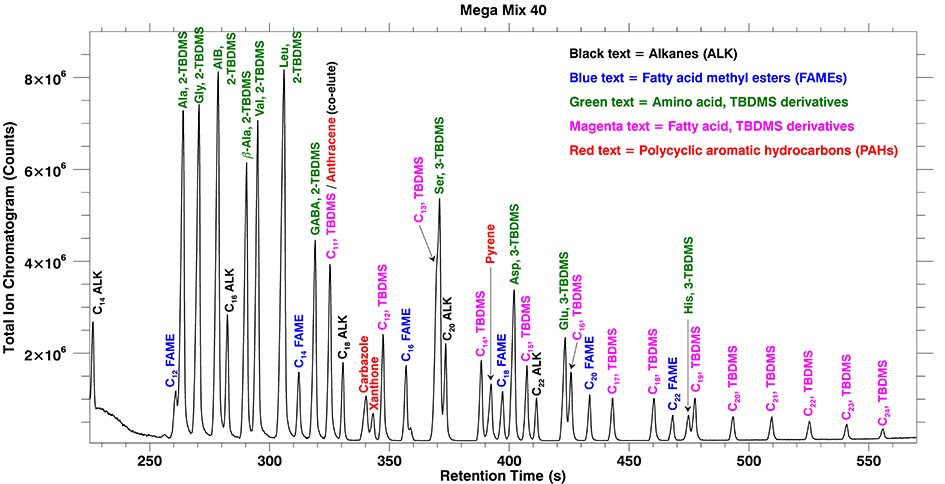
Figure 1: Chromatogram of a complex mixture (40 compounds) including alkanes, chemically derivatized amino and fatty acids, and PAHs separated on a MEMS GC column in less than 10 minutes.
The goal of this Targeted IR was to advance the hardware design and field deployment strategy of the MEMS GC technology in collaboration with Johns Hopkins University Applied Physics Laboratory (JHU APL) for the submission of a proposal to the NASA Planetary Science and Technology through Analog Research (PSTAR) program. JHU APL provides front-end sample handling and cleanup with the use of a Wet Lab for salt removal from an aqueous solution for amino acid purification.
Approach
To demonstrate salt removal (and other unwanted sample matrix effects removal) from an aqueous sample of amino acids, JHU APL employed their Wet Lab with ion exchange chromatography column that we collaborated with in the past through the ICEE 2 program. The demonstrated removal of salts followed by separation of chemically derivatized amino acids (CDAAs) on commercial GC systems and the MEMS GC system was the main goal of this work and was to be used in the submission of our NASA PSTAR proposal (SwRI, JHU APL, and UM team).
For the MEMS GC test, we planned to use a MEMS preconcentrator, a MEMS GC column, and a micro-photoionization detector (μ-PID) for the experimental setup. The MEMS preconcentrator serves to trap and desorb sampled vapor of chemically derivatized amino acids (CDAAs). The desorbed CDAAs are then swept with helium carrier gas to the MEMS GC separation column where they are separated according to their boiling point and affinity for the stationary phase coated on the MEMS GC separation column walls. After eluting from the MEMS GC separation column, the CDAAs would be detected by the μ-PID. A single electronics board designed during the ICEE 2 program would control all components of the MEMS GC system. For the sampling, desorption, separation, and detection of the CDAAs all the MEMS GC components must be closely coupled to minimize cold spots (thermal loss) resulting in condensation of the CDAAs and to minimize dead volume (Figure 2).
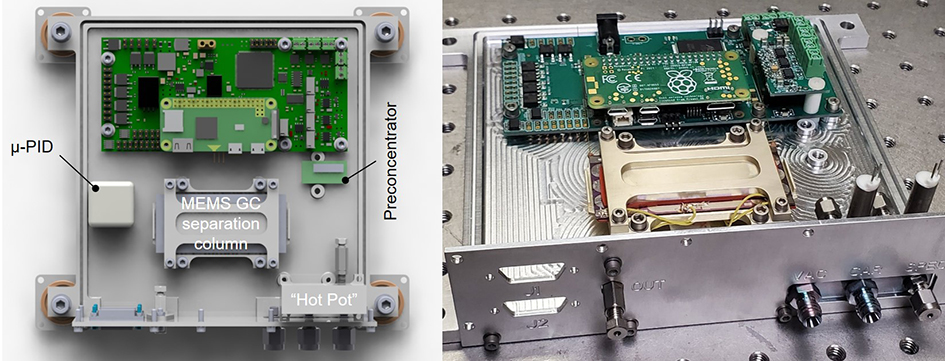
Figure 2: CAD model of the proposed MEMS GC system for experimental testing (left panel) and a prototype box enclosure (without lid) showing the physical hardware with manifold and valves (sampling), MEMS GC separation column inside holder, and the electronics board for control of the experiment (right panel).
Accomplishments
For the salt removal experiment, four amino acids (Glycine [Gly], Serine [Ser], Aspartic Acid [Asp], and Glutamic acid [Glu]) were dissolved in a 600 mM NaCl solution. This solution was run through the JHU APL Wet Lab where amino acids were bound in the ion exchange chromatography column; salts were removed, followed by amino acid elution after salt removal. A portion of the eluted amino acid solution was evaporated to dryness and re-dissolved in distilled water to measure conductivity. The measured conductivity value corresponded to a NaCl concentration of 1-6 mM (according to a standard curve) demonstrating ≥ two orders of magnitude reduction in salt concentration. This effective sample handling and cleanup is paramount to detecting trace species in future landed space missions that provide information on habitability and the search for life elsewhere. A second portion of the eluted amino acid solution was evaporated to dryness, re-dissolved in MTBSTFA, and heated at 90 °C for 1 hour for chemical derivatization of the four amino acids. Analysis of the chemically derivatized amino acids (CDAAs) on commercial GC-MS systems was performed at JHU APL and SwRI. The presence of all four amino acids completely derivatized with tert-butyldimethylsilyl (TBDMS) derivatives at the active sites was observed in the chromatograms and the mass spectra matched the expected mass spectra from the NIST database.
The electronics board designed during ICEE 2 was also configured under this program. The Raspberry Pi was fully configured to run the software followed by testing of the electronics board to control all outputs for the MEMS GC system. A block diagram of the board is displayed in Figure 3 with labels for which MEMS GC components are controlled
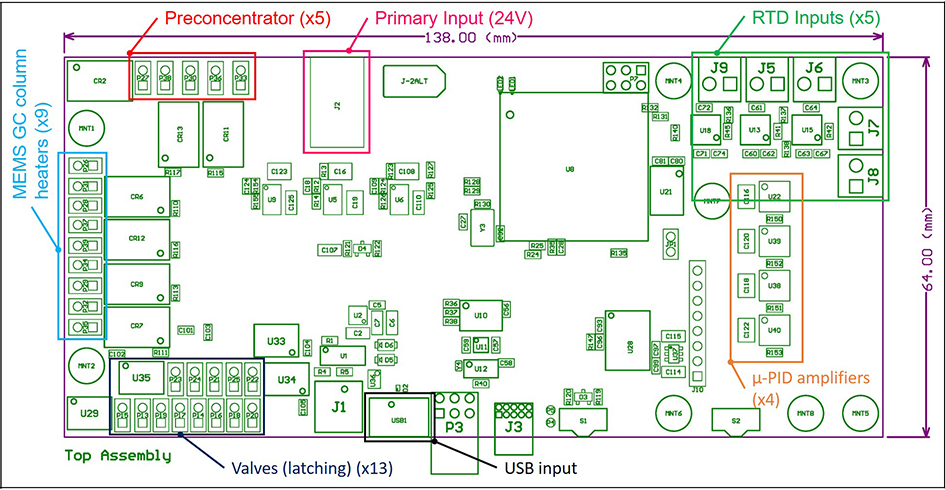
Figure 3: Block diagram of the electronics board to control the components of the MEMS GC system.
Multiple outputs are available for each MEMS GC component on the electronics control board. This allows for multiple sampling pathways and different MEMS GC components such as preconcentrator trapping material, MEMS GC separation column stationary phases, and different micro-detectors for broad chemical selectivity (Figure 4). This is especially advantageous in covering a wide variety of molecules over a range of concentrations from major components at percentage levels to trace level species at parts per billion (ppb) levels.
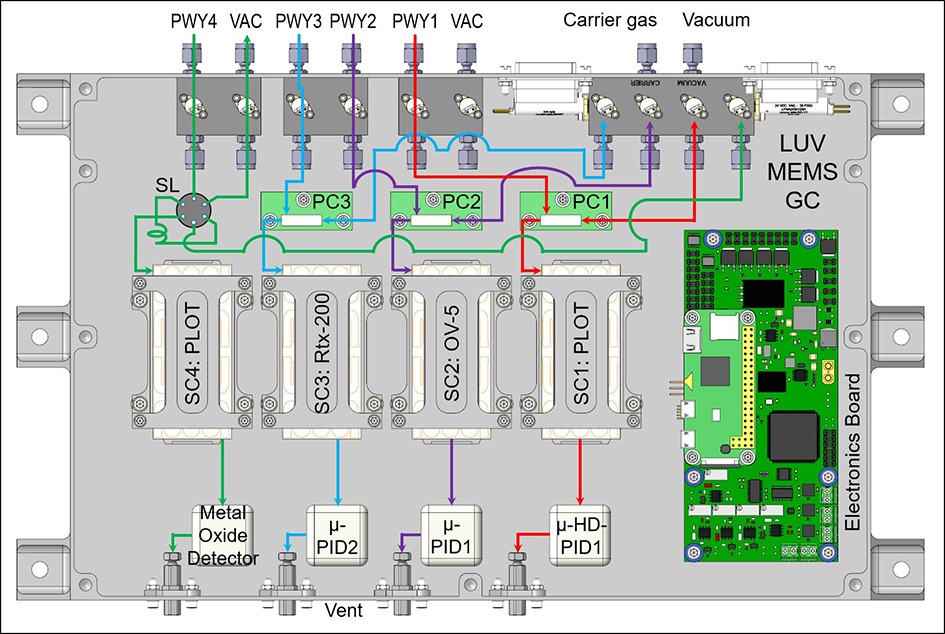
Figure 4: CAD design layout of a multiple MEMS GC column configuration for a future planetary landed mission. This multiplicity allows for targeting different analytes within each MEMS GC separation column to provide broad chemical detection from fixed gases and high volatility species to larger organics and chemically derivatized amino and fatty acids.
