Background
Hydrogen-fueled internal combustion engines (H2-ICE) have rapidly become a key element of the greenhouse gas (GHG) compliance portfolio of engine and vehicle manufacturers around the world. While H2-ICE is highly effective in addressing GHG emissions, any combustion system still generates oxides of nitrogen (NOx) which are local air pollutants related to human health concerns. Modern exhaust aftertreatment technology is highly effective at reducing these NOx emissions to extremely low levels, but the technology is tightly tied to the chemistry of fossil fuel combustion. To allow H2-ICE to be both a zero GHG and near-zero NOx emitter, R&D activity was necessary to develop the fundamental knowledge of how to implement aftertreatment technology for these engines.
Approach
This IR project focused on bench-scale evaluations of a wide range of aftertreatment technologies. These encompassed all the gas-phase aftertreatment stages that are required for an engine: oxidation catalysts to oxidize hydrogen, ammonia, hydrocarbons, and carbon monoxide (these last two can still be released from any engine lubricant that is consumed during combustion) and reducing catalysts to reduce NOx (urea selective catalytic reduction and hydrogen catalytic reduction). Both commercially available catalyst formulations and formulations identified in the literature (which were synthesized at SwRI) were evaluated for their performance in reducing NOx emissions in simulated H2-ICE exhaust on a universal synthetic gas reactor bench. These results were then used to conceptualize an optimized H2-ICE aftertreatment system design.
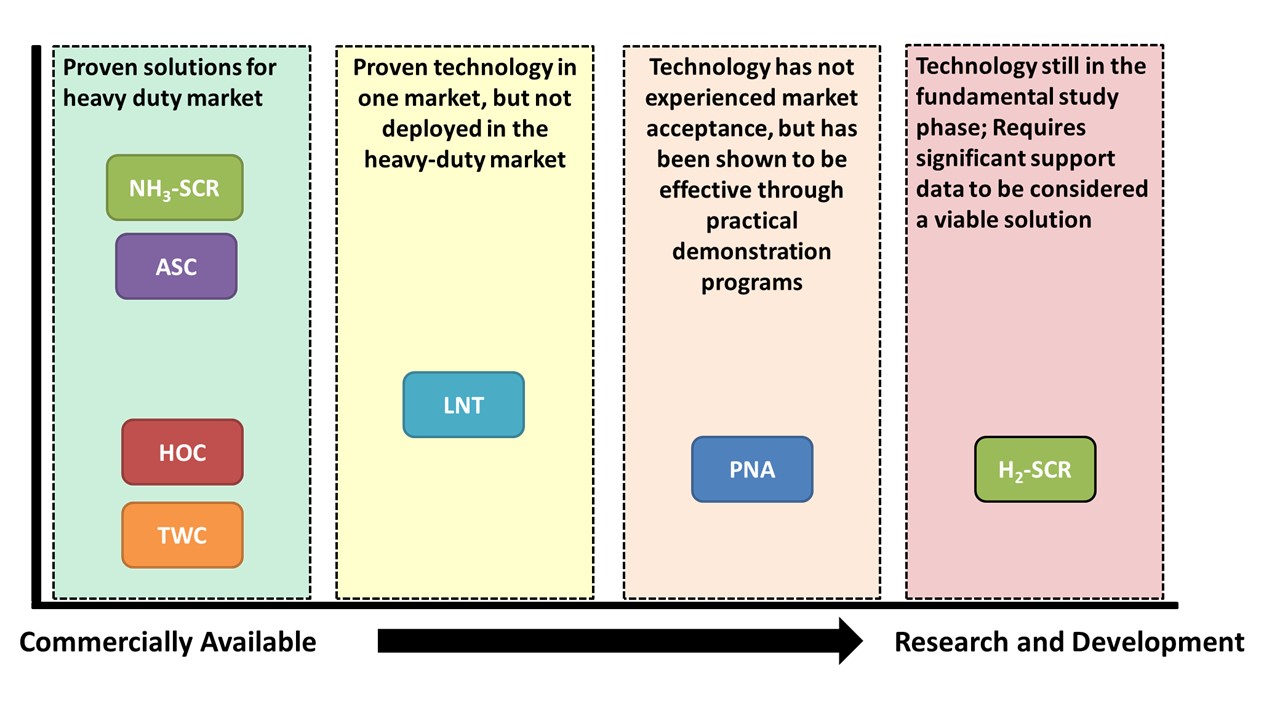
Figure 1: Technology Matrix for Potential H2-ICE Aftertreatment Solutions
Accomplishments
A range of catalyst technologies covering both production and advanced systems was evaluated in a bench reactor to quantify the performance of each technology for hydrogen engine exhaust (see Figure 1). Among the results of interest, it was found that while an oxidation catalyst does generate N2O in the presence of hydrogen, the hydrogen causes an exotherm on the catalyst that rapidly heats the system beyond the temperature where N2O formation occurs, limiting the production of this undesirable species (Figure 2). At the same time, the higher water content of hydrogen engine exhaust does reduce the conversion efficiency of a copper zeolite SCR at low temperatures but shows no impact on the catalyst performance at fully warm temperatures (Figure 3). These results have supported the development and demonstration of a production-intent aftertreatment system that can surpass the emissions performance of a 2027 diesel engine.
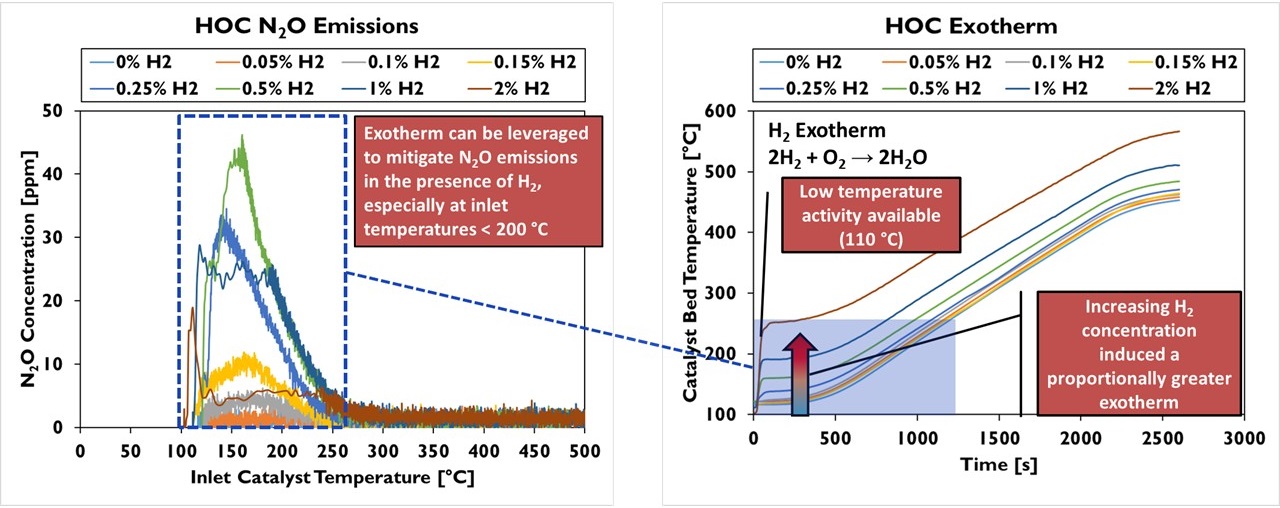
Figure 2: Oxidation Catalyst Response for H2-ICE Exhaust – N2O Formation and Low-Temperature Exotherms
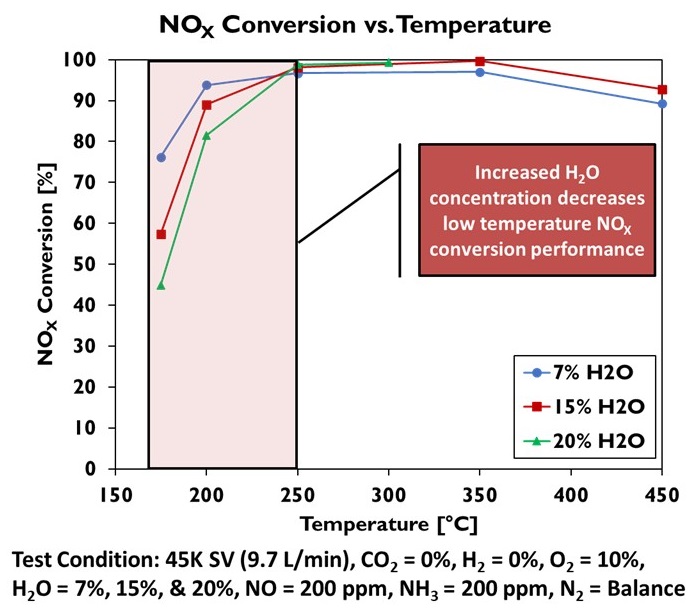
Figure 3: Impact of Increased Water Concentration in Exhaust from H2-ICE Operation on SCR Conversion Efficiency

